EASY
Earn 100
Reaction occurs at anode in the electrolysis of fused is
(a)Chloride ions are oxidized.
(b)Sodium ions are oxidized.
(c)Chloride ions are reduced.
(d)Sodium ions are reduced.
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
EASY
MEDIUM
HARD
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
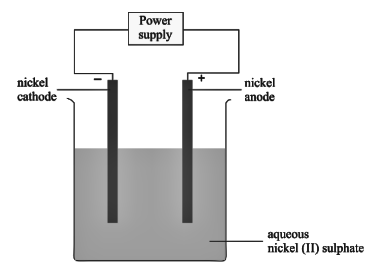
- What do you observe at the cathode and anode respectively?
EASY
HARD
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
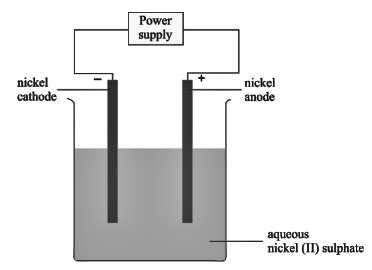
- Which equation for the reaction at the anode is correct?
EASY
Which one of the following statements is correct for electrolysis of brine solution?
EASY
EASY
EASY
MEDIUM
EASY
EASY
HARD
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
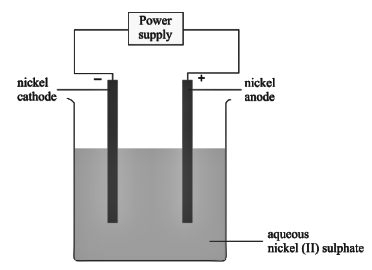
- Name the cation that remains as a spectator ion in the solution.
EASY
MEDIUM
EASY
EASY
The of the solution _________ during the electrolysis of dilute aqueous solution of
EASY
MEDIUM

